SAN ANTONIO — The FDA's approval of Pfizer's coronavirus vaccine on December 11, 2020, kicked off a new phase in the local response to the COVID-19 pandemic. For the first time, there would be a way for people to protect themselves from coronavirus transmission.
Since that time, the FDA also has approved the Moderna vaccine, Johnson and Johnson vaccine, and is continuing to evaluate other vaccine products for use with the general public.
Under Texas state health guidelines, COVID-19 vaccine distribution is being offered in phases. This has prompted many questions about the vaccine's distribution, accessibility, effectiveness and more.
How many people have received the COVID-19 vaccine?
The following numbers are provided by San Antonio Metro Health. A full breakdown can be found here.
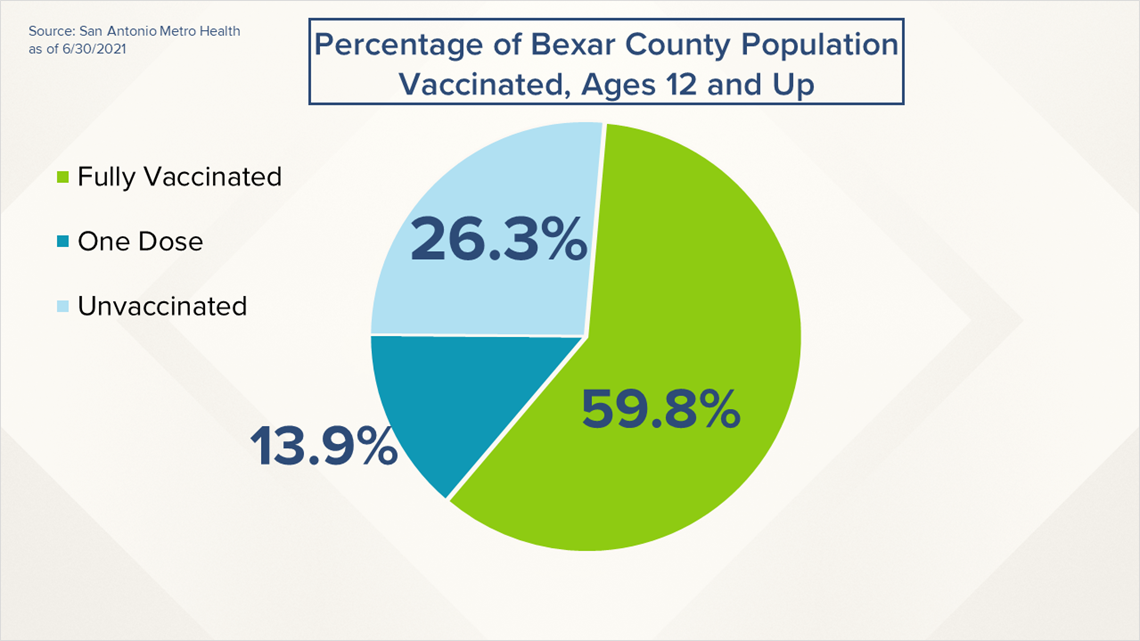
- 1,224,955 Bexar County residents have received at least one dose of the coronavirus vaccine, representing 73.7 percent of the county's population eligible to receive a vaccination.
- 993,844 Bexar County residents are fully vaccinated, representing 59.8 percent of the county's population eligible to receive a vaccination.
- 231,000 Bexar County residents have not yet received their second vaccine dose.

Who can get the COVID-19 vaccine right now?
As of March 29, all adults in Texas are now eligible to receive the vaccine. Those who are 80 and older will be prioritized however and may be moved to the front of the line, officials said. The change was announced March 23.
On May 10, the FDA expanded the emergency use authoritization for the Pfizer vaccine to kids and teens aged 12-15. A CDC panel met on Wednesday, May 12 to issue guidance on the vaccinating kids in that age range. Anyone 12 and older can get the Pfizer vaccine wherever it is offered.
Where can I get the COVID-19 vaccine?
Most vaccinations will be done by appointment by various providers around San Antonio and statewide. KENS 5 will update this tracker with information about providers who have announced that they have appointments available:
- Alamodome: Currently, the Alamodome is serving as a no-cost mass COVID-19 vaccine site. Starting Friday, April 23, anyone 16 and older can get a vaccination between noon and 5 p.m., until supplies last. Click here for more information.
- UT Health San Antonio: The organization began offering COVID-19 vaccine appointments to the public on March 10. The signup requires you to register a new account with UT before you can book an appointment. Click here to create a new account and book an appointment.
- CVS: San Antonio is one of several cities in Texas that has the COVID-19 vaccine available at select CVS locations. And you can now walk into some CVS pharmacies to get a COVID-19 vaccine shot. CVS Health announced on May 5 that it is now accepting walk-in COVID-19 appointments at CVS Pharmacy locations across the country, including 800 locations in Texas. You can visit CVS' website or contact them at 1-800-746-7287 for more information.
- H-E-B: The grocery/pharmacy chain opened up online scheduling for appointments. Check back at the online scheduling page for added appointments in the future.
- Walgreens: The pharmacy is now offering COVID-19 vaccines. Click here for more information or to schedule an appointment.
- Health care providers: Check with your own doctor's office, pediatrician's office or other health care provider to see if they are offering the vaccine patients.
As of Monday, May 10, the FDA expanded the emergency use authorization for the Pfizer vaccine to kids and teens aged 12-15. A CDC panel met on Wednesday to issue guidance on vaccinating kids in that age range. Anyone 12 and older can get the Pfizer vaccine wherever it is offered.
Most places offering the Pfizer vaccine have publicly invited those age 12 and over to receive the shot.
In addition, the following area providers are listed as having received vaccine shipments from the state. Contact directly for potential distribution information:
- Bandera Family Health
- Caritas Family Medicine PA
- Christus Promptu Urgent Care
- Christus Santa Rosa Family Health Center
- Christus Santa Rosa Hospital Alamo Heights
- Davila Pharmacy
- Endeavor Clinical Trials
- Foundation Surgical Hospital Of San Antonio
- Gruesbeck Medical Clinic
- H-E-B Pharmacy (10, 106, 108, 164, 178, 189, 191, 195, 205, 211, 224, 230, 235, 26, 262, 294, 372, 384, 385, 389, 395, 397, 398, 427, 444, 463, 466, 480, 494, 555 556, 566, 567, 568, 585, 618, 623, 647, 658, 678, 699, 732, 733, 771, 84, 85)
- Hormazd Sanjana MD
- Methodist Specialty And Transplant Hospital
- Methodist Stone Oak Hospital
- Northeast Methodist Hospital
- Quality Urgent Care
- Quality Urgent Care Of America
- SAFD Office of Medical Director
- SAMHD Main Immunizations Clinic
- San Antonio Arthritis Care Centers
- South Texas Allergy and Asthma Medical Profession
- Southwest General Hospital
- St. Philip's College
- START Center For Cancer Care
- Steven A Davis MD
- Stone Oak Family Doctors
- UHS Family Health Clinic
- UHS Naco Perrin Clinic 402
- UHS North
- UHS Southwest
- University Health System
- University Of Texas Medicine 8
- University Of Texas Medicine Hill Country
- UT Health San Antonio Verde Hills
- UT Health Shavano Park PCC
- UT Health Westover Hills Clinic
- UT Medicine
- Westover Hills Family Health
Which vaccines have been approved for use?
Pfizer-BioNTech Vaccine
The vaccine by Pfizer and German partner BioNTech is the first of two vaccines that have been approved to fight the coronavirus, which has already claimed more than 300,000 lives in the U.S. and 1.5 million lives globally, per Johns Hopkins University.
According to the U.S. Food & Drug Administration, the Pfizer-BioNTech vaccine was issued an emergency use authorization (EUA) on December 11.
On December 12, the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) met to go over the FDA's recommendation that the agency issued a EUA for the vaccine.
The CDC advisory committee ultimately voted and approved the recommendation to distribute the Pfizer COVID-19 vaccine.
The Pfizer vaccine has been approved for adults and teenagers 16 years of age and older and began distribution in the United States on December 14.
READ MORE:
Moderna Vaccine
On December 18, the U.S. added the Moderna COVID-19 vaccine to its arsenal.
Moderna was the second company to apply with the U.S. Food and Drug Administration for an emergency use authorization for its vaccine.
During the week of December 18, Moderna announced it was prepared to ship 6 million doses of the vaccine. More than 600,000 doses initially were sent to Texas, according to health officials.
On December 23, 500 doses of Moderna's vaccine arrived in San Antonio. They were allotted for health care professionals.
The initial shipments of Moderna’s COVID-19 vaccine were more than double the 2.9 million doses the U.S. prepared to initially ship for Pfizer’s vaccine.
Moderna expects to have between 100 million and 125 million doses available globally in the first three months of 2021, with 85 million to 100 million of those available in the United States.
AstraZeneca Vaccine
The third company to put a vaccine through trial phases is AstraZeneca in partnership with Oxford University. It is unclear when the company plans to apply for emergency use with the FDA. On March 22, news came out that the company may have used outdated information in relation to a trial going in the U.S.
Novavax Vaccine
500 San Antonians are needed for the latest COVID-19 vaccine clinical trial by Novavax. It began in January and is being conducted by UT Health San Antonio and its clinical partner, University Health.
FUJIFILM Diosynth Biotechnologies Texas has an expanded facility in College Station for large scale production of Novavax’ vaccine candidate. Click here to read more about the College Station facility.
Johnson & Johnson Vaccine
On Feb. 26, the Food and Drug Administration’s scientists confirmed that overall the vaccine is about 66% effective at preventing moderate to severe COVID-19, and about 85% effective against the most serious illness. The FDA approved the vaccine for emergency use on Saturday, Feb. 27. The agency also said J&J's shot — one that could help speed vaccinations by requiring just one dose instead of two — is safe to use.
However, on April 13, the FDA and CDC recommended a pause on the Johnson & Johnson vaccine after some cases of rare blood clots. City officials said anyone scheduled to receive a J&J shot would have the option of getting a Moderna or Pfizer vaccine instead.
What's the difference between the available vaccines?
The two vaccines (Pfizer and Moderna) are largely similar:
- Data shows both are between 94 to 95% effective.
- Both require two doses.
- Neither is a live virus vaccine.
- Neither impacts your body's DNA.
- Reported side effects for both are nearly identical: mild fever, headache, fatigue, pain at the injection site.
- Both use new vaccine technology called mRNA.
However, there are some differences to note between the Pfizer and Moderna vaccines:
- Dose timeline: Pfizer's vaccine requires your booster shot three weeks (21 days) after the first. Moderna's booster comes four weeks (28 days) later.
- Age limitation: Pfizer's vaccine was granted emergency use for people ages 16 and older. Moderna's, if approved, will be available for people ages 18 and up.
- Storage: Pfizer's vaccine has to be stored in super cold temperatures (-112 to -76 degrees Fahrenheit). Moderna's parameters are different (-13 to -5 degrees Fahrenheit).
It is important to note that some coronavirus vaccines will need a second dose from the same manufacturer, so people will get a reminder card to keep track of the information.


Experts say the need for two doses of the Pfizer and Moderna vaccines is to help the body build up a defense against the virus.
Dr. Amesh Adalja, an infectious disease specialist offered the following explanation, "We know that there is some immunity that people will get after a single dose, but it may not be as durable, it might not last as long, and it may not be as strong as when you get two doses."
For people who get a Pfizer vaccine, the second dose will come three weeks after the first shot.
Moderna's booster comes four weeks later.
When you get the first dose of either vaccine, you'll be given a vaccine card reminding you to get the second dose, which must be the same vaccine as the first dose.
It is important not to mix doses from different makers. It's also important to note that not getting the second dose could limit the effectiveness of the vaccine.
How important is the second dose?
Experts say the booster shots help increase your immunity against the coronavirus. So what happens if you miss the second dose?
"Nothing happens per say, but so far, we would like people to continue to take that second dose if possible. Because that really offers the highest level of efficacy," Dr. Bryan Alsip, University Health.
But, Dr. Alsip said not to stress if you cannot get your second dose within that time frame.
"The goal would be to get it as soon as possible. Like most vaccines you can take the second dose longer than the interval indicates. But since the studies really were done based on the 21 and 28 day interval, it's important to try and get that dose as close as possible," Dr. Alsip said.
The bottom line is that Dr. Alsip says to make sure you get the second dose as soon as you can for full immunity and make sure it is the same vaccine as your first dose.
Which groups of people should be cautious about the coronavirus vaccine?
Even before the vaccine was approved we heard about who should get a coronavirus vaccine. But it turns out it may not be for everyone.
After the first doses of the coronavirus vaccine were administered there were several reports from around the world of people developing allergic reactions.
Some of the more common vaccine reactions that have been reported include a headache muscle aches, chills, or a fever.
"Our recommendation is, if you've had a severe reaction to an immunization or food allergies where it's been severe enough, you've needed an EpiPen. Then it's an individual decision between you and your doctor whether or not you should get this vaccine," said Dr. Robert Leverence, the Chief Medical Officer at UT Health. He also says another group that should consult with their doctor before getting a COVID vaccine that we don't hear a lot about, are those with autoimmune disorders.
Dr. Leverence said, "Those are people with already revved up immune systems. And here we're going to inject a vaccine intended to ramp up the immune system. And there's some theoretical concern that that ramping up of the immune system can worsen their autoimmune disorder."
He also told us there's just not enough evidence from the original study to know how the vaccine will affect those with certain health conditions. Again, it is best to talk to your doctor to see if the vaccine is right for you.
And when it comes to getting the coronavirus vaccine after getting COVID-19, here's what the experts have to say:
COVID-19 vaccine and pregnancy
Pregnant women were excluded from the vaccine studies that were conducted for emergency authorization.
However, studies looking at how the COVID-19 vaccine will affect women and their babies are underway right now.
What we do know is that pregnant women are at risk for severe complications from the coronavirus.
Meagan Garibay, an RN with the Comanche County Memorial Hospital in Oklahoma, had a mild reaction to the first dose of the vaccine, but that didn't prevent her from getting the second dose.
"I've got a much higher chance of suffering, severe consequences, or even death from COVID-19.
Dr. Laura Rilet, an OB-GYN Chief at New York-Presbyterian and Weill Cornell Medicine echoed this, saying that the benefits of the vaccines are likely to outweigh the risks of COVID-19.
"The biology of the way this vaccine works does not make us think in any way that this vaccine is going to be any less safe or any less efficacious in pregnancy."
According to a Harvard study, pregnant women who tested positive for the virus did not pass it on to their babies.
The study also found that mothers did not pass along any antibodies, meaning their children could still get sick from the virus, later in life.
As for nursing mothers, there's no data yet on how the vaccine could affect milk production.
READ MORE: The COVID-19 vaccine and pregnancy
Distribution in Texas
Phase 1A
COVID-19 vaccinations began with Phase 1A on December 14.
Phase 1A of vaccine allocation prioritizes frontline health care workers and residents of long-term care facilities.
As of December 25, 126,411 Texans have been vaccinated for COVID-19, with just under half a million doses distributed across the state.
During the week of December 28, first responders also began receiving their first doses of the vaccine.
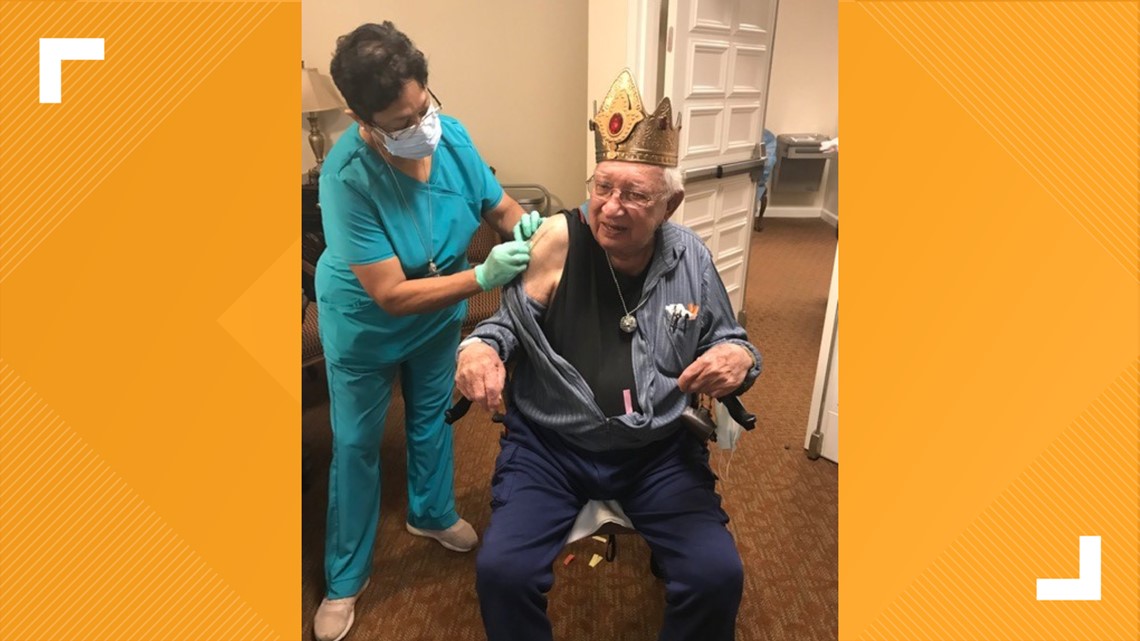
On December 28, San Antonio-area resident Eugene Flores (pictured below) received his COVID-19 vaccination on his 100th birthday.

As of January 28, 2021, more than 2 million cases have been reported in the state, and more than 35,100 people in Texas have died, according to the Texas Department of State Health Services.
On this day the DSHS also confirmed that that of the more than 3 million doses that have been administered in Texas, less than 2,000 have been "wasted."
Phase 1B
On December 29, the Texas Department of State Health Services (DSHS) announced that it would open up vaccinations to Texans in Phase 1B.
DSHS Commissioner John Hellerstedt, MD, released the following statement on vaccine administration in Texas:
“All providers that have received COVID-19 vaccine must immediately vaccinate healthcare workers, Texans over the age of 65, and people with medical conditions that put them at a greater risk of severe disease or death from COVID-19. No vaccine should be kept in reserve.”
Phase 1B includes:
- People 65 years old and older
- People 16 years old and older with at least one chronic medical condition that puts them at increased risk for severe illness from the coronavirus, including but not limited to:
- Cancer
- Chronic kidney disease
- COPD (chronic obstructive pulmonary disease)
- Heart conditions, such as heart failure, coronary artery disease, or cardiomyopathies
- Solid-organ transplantation
- Obesity and severe obesity (body mass index of 30 kg/m2 or higher)
- Pregnancy
- Sickle cell disease
- Type 2 diabetes mellitus
Timeline of vaccine distribution in San Antonio
More than 28,000 doses were expected to be available during the first week of distribution.
December 14: UT Health San Antonio announced the arrival of 6,000 doses of the Pfizer COVID-19 vaccine.
December 16: UT Health San Antonio front-line health care workers, including doctors, nurses, and care team members, were set to receive their first dose of the vaccine.



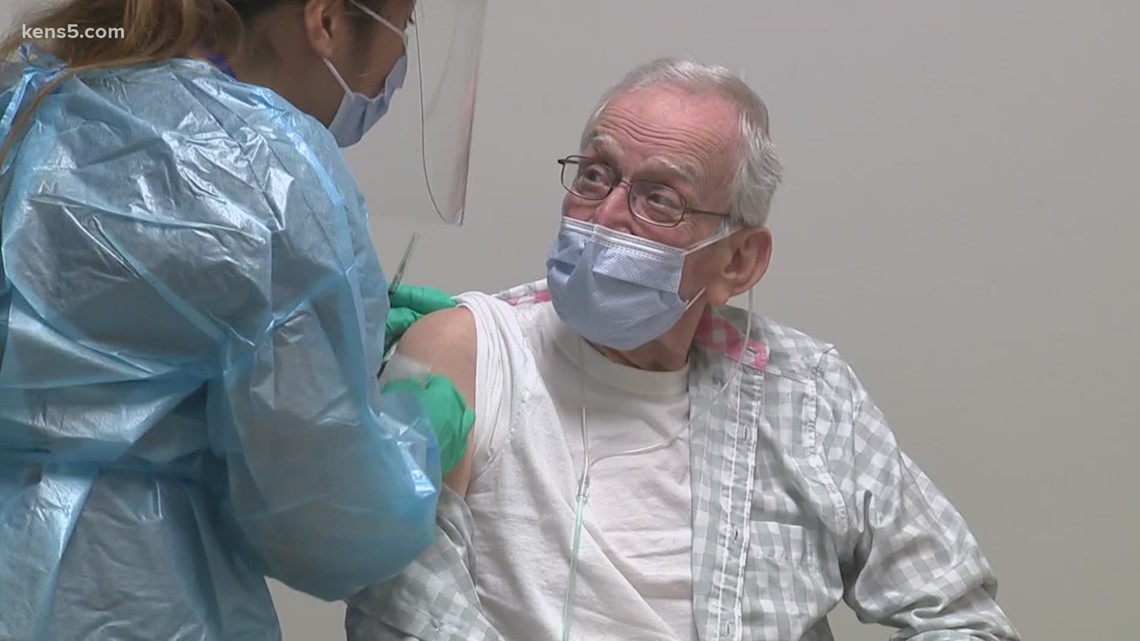
December 16: The San Antonio VA began giving the COVID-19 vaccine to staff members and residents.
December 17: The first shipment of the COVID-19 vaccine arrived at CHRISTUS Santa Rosa Hospital-Medical Center. Doses were administered to associates later that afternoon.
December 17: A total of 11 medical facilities in Bexar County were expected to receive the vaccines during the first week:
- San Antonio State Hospital: 975 doses
- North Central Baptist Hospital: 975 doses
- St. Luke's Baptist Hospital: 975 doses
- Christus Santa Rosa Medical Center: 3,900 doses
- Christus Santa Rosa Hospital - Westover Hills: 4,875 doses
- University Health System - Inpatient: 5,850 doses
- Methodist Hospital & Methodist Children's Hospital: 1,950 doses
- Methodist Metropolitan Hospital: 975 doses
- Northeast Baptist Hospital: 975 doses
- Wellness 360 (Adult): 5,850 doses
- Baptist Medical Center: 975 doses


December 18: During a press conference at the UPS Distribution Center in Austin, Governor Abbott provided an update on the statewide COVID-19 vaccine distribution. The governor shared that during the first week of distribution, 95,000 doses of the vaccine were delivered to 23 different sites throughout the state.
December 21: In Bexar County, more than 35,000 vaccine doses from Moderna and Pfizer were expected to arrive ahead of Christmas.
The following facilities will receive the shipments:
- Bandera Family Health
- Caritas Family Medicine PA
- Christus Promptu Urgent Care
- Christus Santa Rosa Family Health Center
- Christus Santa Rosa Hospital Alamo Heights
- Davila Pharmacy
- Endeavor Clinical Trials
- Foundation Surgical Hospital Of San Antonio
- Gruesbeck Medical Clinic
- HEB Pharmacy (10, 106, 108, 164, 178, 189, 191, 195, 205, 211, 224, 230, 235, 26, 262, 294, 372, 384, 385, 389, 395, 397, 398, 427, 444, 463, 466, 480, 494, 555 556, 566, 567, 568, 585, 618, 623, 647, 658, 678, 699, 732, 733, 771, 84, 85)
- Hormazd Sanjana MD
- Methodist Specialty And Transplant Hospital
- Methodist Stone Oak Hospital
- Northeast Methodist Hospital
- Quality Urgent Care
- Quality Urgent Care Of America
- SAFD Office of Medical Director
- SAMHD Main Immunizations Clinic
- San Antonio Arthritis Care Centers
- South Texas Allergy and Asthma Medical Profession
- Southwest General Hospital
- START Center For Cancer Care
- Steven A Davis MD
- Stone Oak Family Doctors
- UHS Family Health Clinic
- UHS Naco Perrin Clinic 402
- UHS North
- UHS Southwest
- University Health System
- University Of Texas Medicine 8
- University Of Texas Medicine Hill Country
- UT Health San Antonio Verde Hills
- UT Health Shavano Park PCC
- UT Health Westover Hills Clinic
- UT Medicine
- Westover Hills Family Health
A list of Moderna's COVID-19 vaccine allocation in week 2 (the second week of Moderna vaccine; third week of vaccines overall) can be found here.
RELATED: Some areas of Texas begin to vaccinate people 65 and older, people with pre-existing conditions
December 30: H-E-B announced that it would not be taking walk-in appointments for COVID-19 vaccinations. A spokesperson said that appointments will be scheduled with patients at their local pharmacies to ensure that "we use every dose in the vial as to not waste a single dose."
Those in Phase 1B are asked to use H-E-B's online scheduling tool as soon as it is launched, which is expected to be sometime next week.
December 30: San Antonio leaders attempted to address the confusion surrounding who is eligible for a vaccine.
More than 31,000 people have received their first dose of the coronavirus vaccine in Bexar County as part of Tier 1A. Dr. Colleen Bridger, an assistant city manager, stressed that it is vital to immunize first-priority recipients (those who are part of Tier 1A), which is an estimated 140,000 eligible candidates. That includes frontline medical workers, such as hospital employees, EMS responders, long-term care facility employees, and residents, she said.
January 8: City of San Antonio officials announced that the Alamodome would serve as a no-cost mass COVID-19 vaccine site starting on January 11, 2020.
January 9: Registration for appointments at the Alamodome site opened at 9 a.m. By 9:06 a.m. all 9,000 slots were filled. According to an official with CoSA's Office of Emergency Management, 11,000 people were on the site at 9 a.m. It was also announced this day, that the WellMed Elvira Cisneros Senior Community Center on the southside would operate as a similar no-cost vaccination site, however, appointments for this site would need to be made by phone.
January 15: WellMed re-opened its COVID-19 hotline on Saturday, January 16 to schedule appointments for 9,000 more doses.
The toll-free number to schedule that appointment is 833-968-1745. The hotline will be open from 8 a.m. to 8 p.m. daily until appointments are filled.
As a reminder, at this time, health care workers, anyone age 65 and older, and adults 18 and older with chronic health conditions are eligible to be vaccinated.
The Alamodome will also begin scheduling appointments for next week.
The San Antonio Metro Health District received another 9,000-dose shipment of the Pfizer COVID-19 vaccines and will begin booking appointments today for the Alamodome next week.
To avoid the overwhelming rush of residents seeking appointments that occurred with the first series of Alamodome appointments, the City of San Antonio will make appointments available on a rolling basis.
Appointments made today will be for approximately 2,000 slots on Monday; tomorrow, the City will make available 2,000 slots for Tuesday and so on.
The registration system will continue to reopen as appointments become available.
Appointments for the Alamodome can be made by visiting this website.
Those without internet access may call 311 and select option 8 to book an appointment. A busy signal indicates that all operators are taking other calls, and callers should try again.
January 22: Citing high demand, University Health officials said they would open up their vaccination schedule and allow residents to try locking down appointments more than one week in advance. More information can be found here.
RELATED: Meanwhile, over in Comal County, officials are trying to ramp up vaccine efforts, but more doses are needed.
January 23: It was announced that Texas would receive 332,750 first doses of the COVID-19 vaccine from the federal government during the week of January 25.
The Texas Department of State Health Services has instructed the Centers for Disease Control and Prevention to ship those doses to 212 providers across Texas. That includes 82 hub providers that will focus on larger community vaccination efforts and 130 additional providers as Texas continues to vaccinate health care workers, residents of long-term care facilities, people 65 and older, and those with medical conditions that put them at greater risk of hospitalization and death from COVID-19.
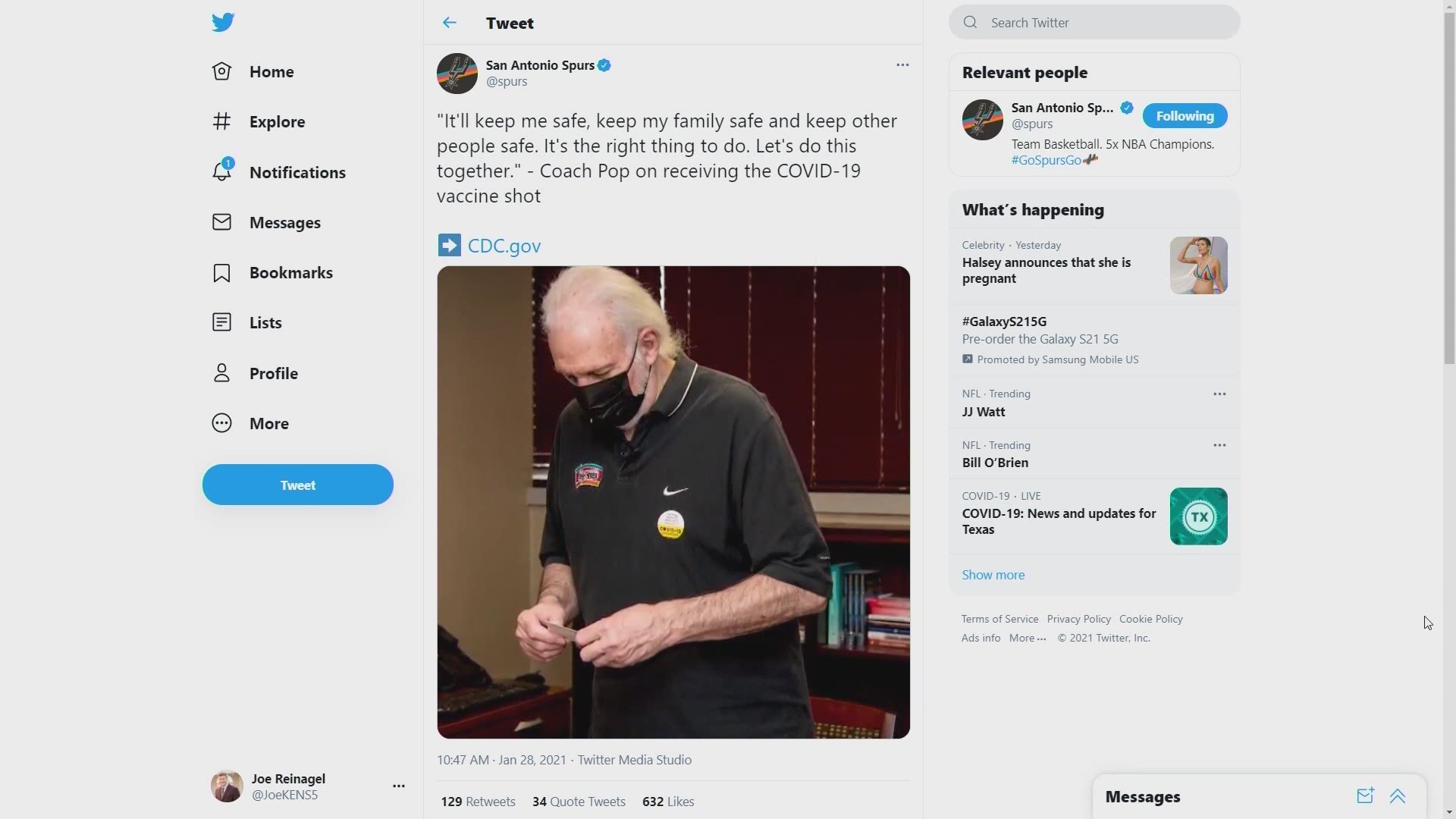
January 28: Spurs coach Gregg Popovich gets the COVID-19 vaccine, saying "It'll keep me safe, keep my family safe and keep other people safe. It's the right thing to do." He urges others to do the same.
January 28 (continued): During a press conference this morning, it was announced that more than 2 million cases have been reported in the state, and more than 35,100 people in Texas have died, according to the Texas Department of State Health Services.
On this day the DSHS also confirmed that that of the more than 3 million doses that have been administered in Texas, less than 2,000 have been "wasted."
February 4: San Antonio will be one of several cities in Texas that will have the COVID-19 vaccine available at select CVS locations. The pharmacy company will begin to administer COVID-19 vaccines to eligible populations on Thursday, February 11 at 70 CVS locations in Texas. Appointments will become available for booking as early as Feb. 9. Supply for the limited rollout in the state, which is sourced directly from the federal pharmacy partnership program, will be approximately 38,000 total doses
February 5: WellMed says it will begin to distribute second doses of the Modern vaccine beginning Monday, Feb. 8, at two San Antonio clinics. Those who received their first immunization through WellMed will be notified about plans to return for a second shot.
February 9: The vaccine appointment hotline reopened at 8 a.m. on Tuesday, February 9 for the WellMed Elvira Cisneros Senior Community Center and WellMed Alicia Trevino Lopez Senior One-Stop Center. Please call 833-968-1745 to book an appointment. The hotline will stay open between 8 a.m. to 8 p.m. again until all available doses of the COVID-19 vaccine have been scheduled.
February 11: San Antonio will be one of several cities in Texas that will have the COVID-19 vaccine available at select CVS locations. Appointments will become available for booking Thursday, Feb 11. The pharmacy company will begin to administer COVID-19 vaccines to eligible populations on Friday, Feb. 12 at 70 CVS locations in Texas.
February 23: WellMed clinics announced it would reopen its vaccination hotline at 8 a.m. on Wednesday, Feb. 24, in order to fill 30,000 first-dose appointments. As a reminder: there is not other method being offered by WellMed to make an appointment.
March 4: WellMed says it will reopen its hotlines at 8 a.m. on Friday, March 5, to fill 9,000 appointments for first doses of the Moderna vaccine at its sites.
March 24: City officials say they will open 30,000 additional coronavirus vaccination slots on Thursday, March 25, at 7 p.m. Those appointments will be for between April 6 and May 1, and they can be made on Metro Health vaccine registration website. Those without internet access can try to lock down an appointment by calling 311.
April 10: WellMed is opening appointments for more than 3,000 doses of the Johnson & Johnson/Janssen COVID-19 vaccine at the Doris Griffin Senior One-Stop Center. The appointments will take place for two days from Monday, April 12 to Tuesday, April 13 from 8 a.m. to 4 p.m. on each day. You can register at this website.
May 10: The FDA expanded the emergency use authoritization for the Pfizer vaccine to kids and teens aged 12-15. A CDC panel plans to meet on Wednesday, May 12 to issue guidance on the vaccinating kids, and medical centers are expected to begin offering the vaccine to that age group sometime after the guidance issued.
May 12-The CDC issued guidance on distribution of the Pfizer vaccine for kids and teens ages 12-15 years old. Those in that age range can get the vaccine anywhere Pfizer is offered.
How vaccines work
The best way to combat misinformation is to understand the facts. So, here are the facts regarding vaccines:
According to the Centers for Disease Control and Prevention, a vaccine stimulates your immune system to produce antibodies, exactly like it would if you were exposed to the disease. After getting vaccinated, immunity is developed to the disease.
While vaccines contain the same germs that cause the disease, the germs have either been killed or weakened to the point that they don't make you sick.
"These leading vaccine trials don't have the live virus in them," said Dr. Linda Nabha, an infectious diseases specialist. "So you can't, for example, get COVID from these vaccines that don't have the live virus in them."
That fact was backed up by other experts.
"You cannot get COVID from the vaccine itself because the vaccine doesn't include the full COVID virus," said Dr. David Diemert, an associate professor of medicine at the George Washington University. "So it's not possible for the vaccine to suddenly make the virus in the person's body."
Achieving herd immunity
Herd immunity is a term used to describe the point at which enough people are protected that the virus can be held in check. The percentage of the population required to develop herd immunity varies with each disease, according to the World Health Organization. Experts estimate at least 70% of the U.S. population needs to be vaccinated to achieve herd immunity.
Measles requires 95% of the population to be vaccinated, with the remaining 5% protected by those who are vaccinated and not spreading the virus.
Slaoui has estimated the country could reach herd immunity as early as May, based on the effectiveness of the Pfizer and Moderna vaccines.
The vaccine and the economy
As more members of the community continue to receive the vaccine, there is hope that the vaccine will help businesses get back to normal and ease the strain on the economy.
Venkatesh Shankar, PhD, a business professor at Texas A&M, says while the vaccines impact on the economy will be positive, those temporary changes we made in how we work, learn and consume could take hold long term.
Listen to the full conversation in our KENS 5 Commerce Street podcast:
How effective is the vaccine?
Pfizer has reported its vaccine to be 94.5% effective in clinical trials. Moderna has said its vaccine is 95% effective.
The vaccine from AstraZeneca and Oxford University suggest the vaccine is about 70% effective. Still, experts say the vaccine seems likely to be approved, despite some confusion in the results and lower levels of protection than what some other vaccine candidates have shown.
Side effects of the vaccine
There will be some side effects, said Dr. Matthew Woodruff, an immunologist at Emory University who studies the fundamentals of immune responses to vaccination.
He writes in The Conversation that vaccines work by training your immune system to recognize and remember a pathogen safely. Expected side effects include redness and swelling at the injection site and stiffness and soreness in the muscle.
A potent vaccine may even cause fever, he said. Pfizer said 3.8% of vaccine recipients during the drug trial felt fatigued, and 2% had a headache. Woodruff says this is normal.
"These are signs that the vaccine is doing what it was designed to do – train your immune system to respond against something it might otherwise ignore so that you’ll be protected later. It does not mean that the vaccine gave you COVID-19,” Woodruff said.
There also were some early questions from England after two people who received the vaccine suffered allergic reactions from the Pfizer vaccine. Experts say such reactions are not unexpected and they are usually rare and short-lived.
The vaccine will not give you COVID-19
A question the Vaccine Team has received a lot is whether or not you can get COVID-19 from the coronavirus vaccine.
"You cannot get COVID-19 from these vaccines. You cannot. All right? None of them are live viruses."
That's the short answer from UT Health Chief Medical Officer, Dr. Robert Leverence.
After COVID-19
Dr. Fauci says it is necessary to get the vaccine because it isn’t certain how long natural immunity from the virus lasts. The CDC adds that early evidence suggests natural immunity, from those who’ve had the virus, may not last long. The agency also adds that immunity can vary from person to person.
'Keep the mask'
For a couple of reasons, masks and social distancing still will be recommended for some time after people are vaccinated.
To start, the first coronavirus vaccines require two shots; Pfizer’s second dose comes three weeks after the first, and Moderna’s comes after four weeks. And the effect of vaccinations generally isn’t immediate.
People are expected to get some level of protection within a couple of weeks after the first shot. But full protection may not happen until a couple of weeks after the second shot.
Coronavirus symptoms
The symptoms of coronavirus can be similar to the flu or a bad cold. Symptoms include fever or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, new loss of taste or smell sore throat, congestion or runny nose, nausea or vomiting and diarrhea, according to the Centers for Disease Control.
Most healthy people will have mild symptoms. A study of more than 72,000 patients by the Centers for Disease Control in China showed 80 percent of the cases there were mild.
But infections can cause pneumonia, severe acute respiratory syndrome, kidney failure, and even death, according to the World Health Organization. Older people with underlying health conditions are most at risk.
But infections can cause pneumonia, severe acute respiratory syndrome, kidney failure, and even death, according to the World Health Organization. Older people with underlying health conditions are most at risk.
Experts determined there was consistent evidence these conditions increase a person's risk, regardless of age:
- Chronic kidney disease
- COPD (chronic obstructive pulmonary disease)
- Obesity (BMI of 30 or higher)
- Immunocompromised state (weakened immune system) from solid organ transplant
- Serious heart conditions, such as heart failure, coronary artery disease, or cardiomyopathies
- Sickle cell disease
- Type 2 diabetes
The CDC believes symptoms may appear anywhere from two to 14 days after being exposed.
Human coronaviruses are usually spread...
- Between people who are in close contact with one another (within about 6 feet).
- Through respiratory droplets produced when an infected person coughs, sneezes or talks. These droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs.
- Some recent studies have suggested that COVID-19 may be spread by people who are not showing symptoms.
Help stop the spread of coronavirus
- Stay home when you are sick.
- Eat and sleep separately from your family members
- Use different utensils and dishes
- Cover your cough or sneeze with your arm, not your hand.
- If you use a tissue, throw it in the trash.
Find a Testing Location
City officials recommend getting a COVID-19 test if you experience fever or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, or diarrhea.
San Antonio operates several no-cost testing locations, including two walk-up locations open Monday-Sunday from 10 a.m. until 2 p.m.:
Cuellar Community Center
5626 San Fernando St.
San Antonio, TX 78237
Ramirez Community Center
1011 Gillette Blvd.
San Antonio, TX 78224
Additionally, Freeman Coliseum offers drive-through no-cost testing from Monday through Sunday between 9 a.m. and 4 p.m. An appointment is required and can be made either online or by calling (833) 213-0643.
Here's a Testing Sites Locator to help you find the testing location closest to you in San Antonio.