SAN ANTONIO — Monoclonal antibodies have been touted as successful treatments against COVID-19. However, many of our viewers heard the treatment may be discontinued. We put the VERIFY team to work to find out if this was true.
THE QUESTION
Is the FDA is stopping current monoclonal antibody treatments for those with the omicron variant of COVID-19?
THE SOURCES
- Elliott Mandell, the Head of Pharmacy Services for University Health
- The Food and Drug Administration.
THE ANSWER
WHAT WE FOUND
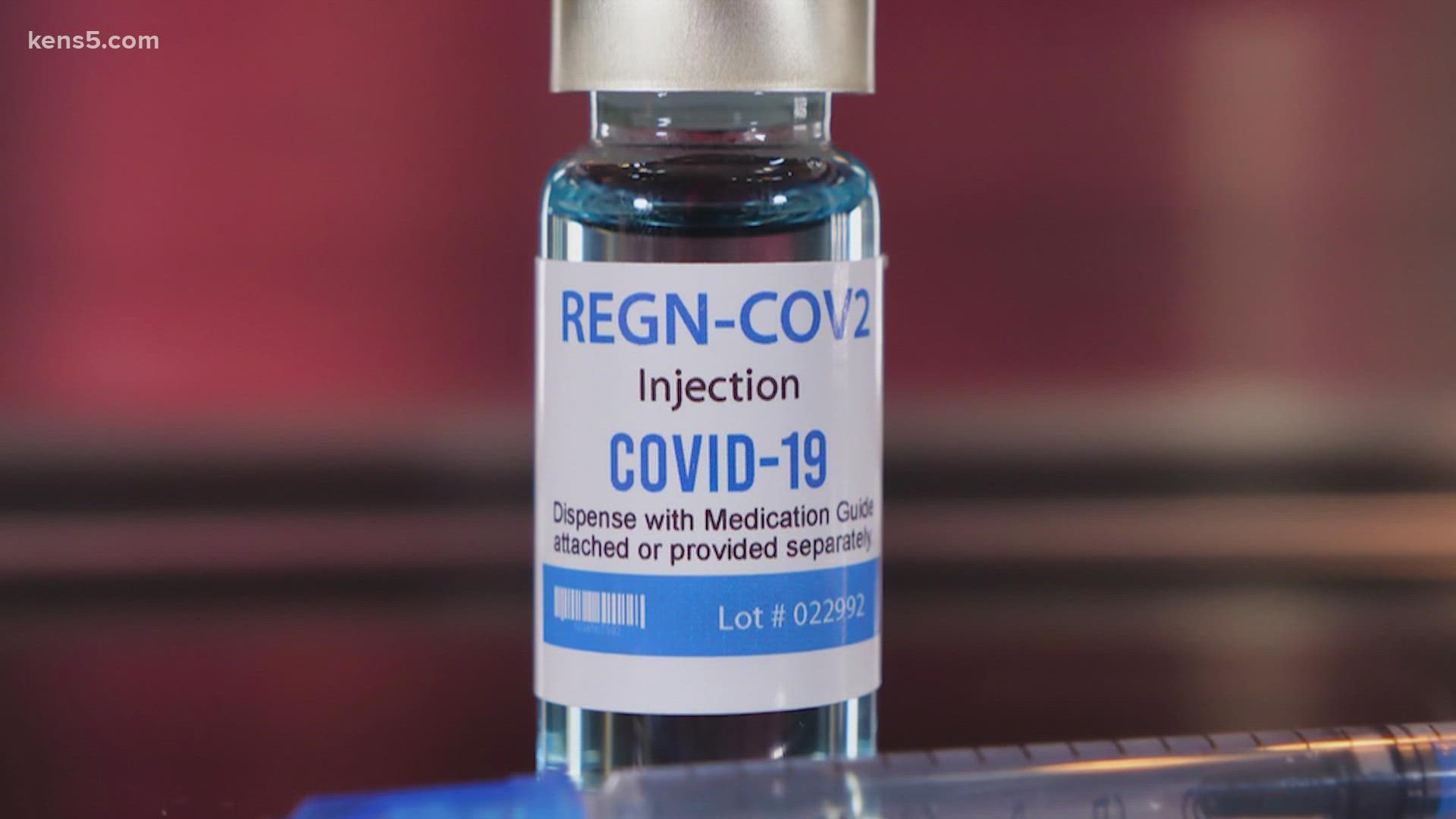
Elliot Mandell, the Head of Pharmacy at University Health said, "As these variants develop, there are different drugs that have effectiveness against it. The drugs that they remove, the emergency use authorization for Bamlanivimab, and the Regeneron product, which was a combination of two monoclonal antibodies that were developed to the treatment of the Delta variant." He says those drugs are not effective against the omicron variant.
In a statement the FDA says, "Because data show these treatments are highly unlikely to be active against the omicron variant, which is circulating at a very high frequency throughout the United States, these treatments are not authorized for use in any U.S. states, territories and jurisdictions at this time."
So yes, it is true, The FDA is stopping current monoclonal antibody treatments for those with the omicron variant of COVID-19.
However there is a new treatment called Sotrovimab that has shown effectiveness against omicron. It just isn't as widely available yet.
Related links: